VZDĚLÁVÁNÍ
Zde najdete informace pro pacienty i pro odborníky ve zdravotnictví.
PRO PACIENTY
Stent
Stent je pletivová trubička fungující jako výztuž pro obnovení průchodnosti zúžené anatomické trubice (trávicí trakt, dýchací cesty, atd.), které je způsobeno benigním či maligním onemocněním nebo poraněním tkáně. Stent je vyroben většinou z nitinolu (sloučenina niklu a titanu, potažený speciálním potahem), který se vyznačuje tvarovou pamětí, elasticitou a odpovídající radiální silou. Jedná se o bezpečnou, standardní a zavedenou metodu.
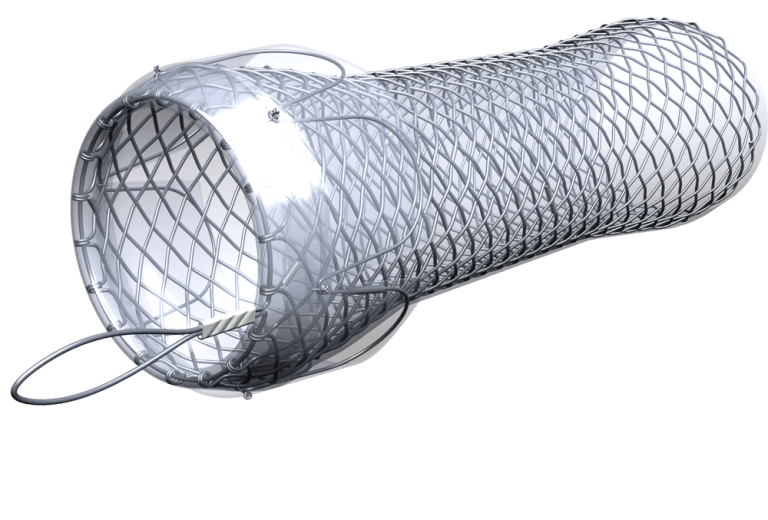
Stent se implantuje na dobu určitou, po které následuje jeho vytažení (extrakce), nebo jako permanentní – trvalé řešení.
Jak se implantuje stent?
Všechny naše stenty jsou samoexpandabilní (samy se rozevírají) a zavádějí se přirozenými tělními otvory, nebo, v případě specifických stentů do žlučových cest v játrech, se mohou zavádět přes přístupový katetr bokem těla.
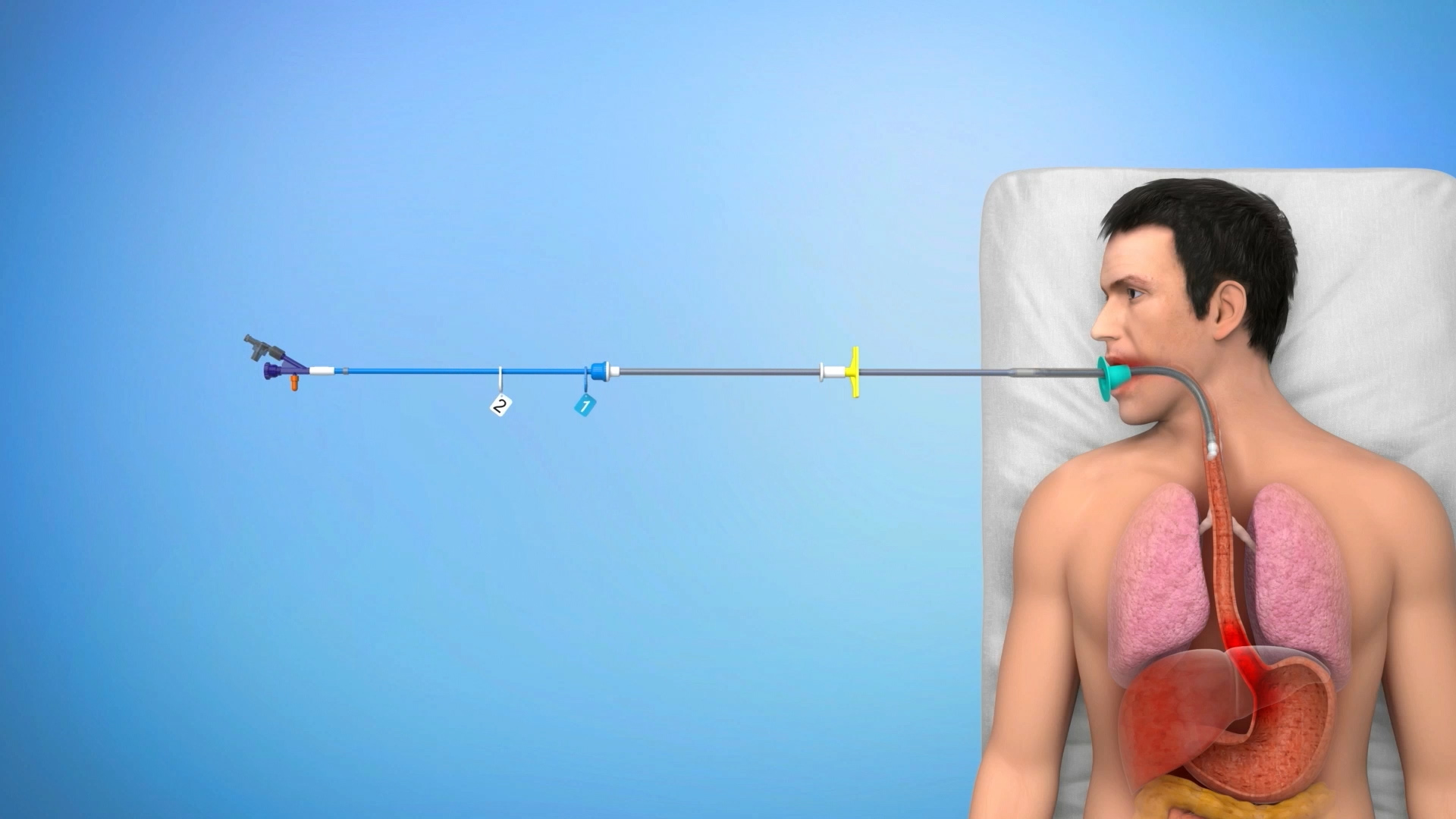
Stentování gastrointestinálního traktu většinou vyžaduje použití endoskopu a rentgenu, nebo alespoň jednu z těchto technik. Postup může být proveden i u vysoce rizikových pacientů, s celkovou anestezií nebo mírnou sedací.
Stentování gastrointestinálního traktu je spojeno s vysokou mírou bezpečnosti a technického úspěchu.
Před implantací stentu
Vyšetření před zákrokem (doporučuje a zajistí ošetřující lékař):
Kontrola srážlivosti – hemokoagulačních hodnot krve. Alergická anamnéza, nachlazení, infekční či jiné imunitní onemocnění. Předchozí radio-chemoterapie. Celkový stav pacienta. Gravidita.
Pokyny pro pacienta před zákrokem:
Přerušení příjmu potravy (lačnění) minimálně 4-6 hodin před zákrokem v jícnu (příjem tekutin dovolen). V případě implantace do tlustého střeva celý den vynechejte pevnou stravu, pijte jen čiré tekutiny, navíc 5 dní před vyšetřením vynechejte potraviny s velkým množstvím nestravitelných zbytků.
Po implantaci stentu
V případě návštěvy jiného odborného lékaře (jiná specializace), prosím, informujte ho předem o zavedeném metalickém stentu (platí především při vyšetření pomocí RTG, CT, NMR, UZ).
Po implantaci jícnového stentu je obvyklý pocit tlaku na hrudi nebo mírné bolesti, které by měly odeznít během cca 1 týdne. V případě jakýchkoliv potíží, setrvávající bolestivosti, hromadění potravy v jícnu, nebo jiných obtíží, kontaktujte bezodkladně svého ošetřujícího lékaře.
Dietní doporučení po implantaci jícnového stentu
Stent se jemně rozpíná a udržuje zúženou oblast jícnu otevřenou, čímž usnadní polykání jídla a nápojů. Stent nebude tak široký ani pružný jako normální jícen, takže budete muset dávat pozor na některá jídla a také na to, jak jíte, abyste zabránili jeho zablokování.
Nejčastějším důvodem zablokování stentu je jídlo, které je spolknuto, aniž by bylo dostatečně rozžvýkáno, nebo potraviny, které se při žvýkání dostatečně nerozkládají. Proto je doporučeno (po celou dobu umístění stentu) všechna sousta dobře rozkousat, nebo jinak rozmělnit a každé sousto zapíjet.
Doporučené pokyny pro stravování (týká se ale především jícnových stentů)
- Důkladně jídlo rozkousejte.
- Pokud je to možné, jezte 5-6 menších jídel denně.
- Jezte pomalu.
- Při jídle seďte vzpřímeně.
- Mezi jídly pijte dostatečné množství tekutin.
- Zůstaňte ve vzpřímené poloze alespoň 30-60 minut po jídle.
- Potraviny by měly být vždy připravovány tak, aby byly vláčné, měkké a snadno se spolkly.
- Pokud někdy cítíte „zaseknuté“ jídlo v krku, zapijte jej trochou perlivé vody. To potravu pomůže uvolnit.
- Pokud máte potíže s udržením hmotnosti, je vhodné používat potravinové doplňky nebo domácí mléčné koktejly. Pokud potřebujete poradit, je vhodné sjednat schůzku s nutričním specialistou.
V případě umístění stentu skrze dolní jícnový svěrač (kardii) neuléhat bezprostředně po jídle, preferovat spíše polohu vsedě. Při spánku je vhodné zajistit zvýšenou polohu horní části trupu a hlavy.
Karta pacienta / Karta implantátu
Zde naleznete aktuální leták se základními informacemi pro pacienta s implantovaným stentem. Pro podrobnější informace kontaktujte implantujícího lékaře uvedeného na Vaší kartě pacienta, nebo svého ošetřujícího lékaře.
| Název produktu | Typ souboru | Název produktu | Typ souboru |
|---|---|---|---|
| BD stent | Karta pacienta | Boubella | Karta pacienta |
| Danis stent | Karta pacienta | Boubella-E | Karta pacienta |
| HV Stent Plus | Karta pacienta | Nitinella Plus | Karta pacienta |
| Flexella Plus PULL | Karta pacienta | Nitinella Plus – B | Karta pacienta |
| Danis Seal | Karta pacienta | Enterella Pyloroduodenální | Karta pacienta |
| ELLA-BD Stent Biliární THP | Karta implantátu | Enterella Kolorektální | Karta pacienta |
Návod k použití
| Název produktu | Typ souboru | ELLA-BD Stent Biliární THP | Návod k použití |
|---|
PRO ODBORNÍKY
Zde naleznete aktuální souhrn publikací o ELLA produktech.
Provádíme také individuální konzultace a produktová školení. Pro bližší informace nás kontaktujte prostřednictvím kontaktního formuláře.
BD Stent
Dilation or biodegradable stent placement for recurrent benign esophageal strictures: a randomized controlled trial
Daisy Walter, Maarten W. Van Den Berg, Meike M. Hirdes, Frank P. Vleggaar, Alessandro Repici, Pierre H. Deprez, Laurence Lovat, Bartolomé L. Viedma, Bas L. Weusten, Raf Bisschops, Renan Haidry, Elisa Ferrara, Keith J. Sanborn, Erin E. O’Leary, Jeanin E. Van Hooft, Peter D. Siersema
Endoscopy. 2018 Mar. Georg Thieme Verlag KG Stuttgart. New York, ISSN 0013-726X
At 3 months, the biodegradable stent group (n = 32) underwent significantly fewer endoscopic dilations for recurrent stricture compared with the dilation group (n = 34; P < 0.001). By 6 months, the groups were similar. The number of patients experiencing adverse events was similar between the groups.Through 12 months, the groups were similar for the EQ-5D composite score (P = 0.57). However, patients in the biodegradable stent group reported a significantly better quality of life through 12 months than patients in the dilation group based on the EQ-5D VAS (P = 0.01). Within the biodegradable stent group, the WHO performance score significantly improved compared with baseline; however, no difference was seen in the dilation group. Through 12 months, the biodegradable stent group showed a significantly higher level of activity as measured by the WHO performance score compared with the dilation group.
The use of reabsorbable ELLA stent in the treatment of benign stenosis
D. Esposito, F. Calabrese, L. Fanti, E. Viale, P. A. Testoni
Abstracts of the 24th National Congress of Digestive Diseases / Digestive and Liver Disease 50/S2 (2018) e63–e238
A total of 20 reabsorbable stents were inserted to 9 patients, one was lost on follow-up, 6 patients had a clinical and endoscopic resolution at the end of follow-up (75%), 1 had a neoplastic relapse and 1 underwent the positioning of a SEMS. The most common adverse event encountered was the formation of granulation tissue creating a substenosis in 2 (10%) patients (successfully treated with another Ella stent insertion). Self limiting bleeding was seen in 1 (5%) patient and 1 (5%) patient complained with pain controlled by mean of analgesics. The insertion of a reabsorbable stent is a safe procedure, with a success rate of 75% but with a multiple number of devices/patient needed since the use of a single stent is seldom sufficient. Such a procedure should be considered as a therapy to be used in repeated sessions similarly to dilation therapy.
Endoscopically placed stents: a useful alternative for the management of refractory benign cervical esophageal stenosis
Nogales Óscar, Clemente Ana, Caballero-Marcos Aránzazu, García-Lledó Javier, Pérez-Carazo Leticia, Merino Beatriz, López-Ibáñez María, Pérez Valderas María Dolores, Bañares Rafael, González-Asanza Cecilia.
Rev Esp Enferm Dig 2017. doi: 10.17235/reed.2017.4795/2016.
A total of 23 stents (13 FCSEMS and 10 BDS) were placed in 12 patients (median 1.92, range 1-4, 6 patients received at least one BD Stent). The technical success rate was 96% (22/23 stents). Eight patients (66.6%) maintained adequate oral intake at theendof follow-up (median 33.3 months, range 3-84 months). All patients complained of minor cervical pain after placement that was well controlled with mild analgesia. Migration was recorded in 7/23 stents (30.4%) and epithelial hyperplasia in4/23 stents (17.4%). Interestingly, migration was observed in 7/13 FCSEMS (53.8%) but not in BDS (0%; p = 0.005) cases. All the migrated FCSEMS were successfully repositioned using endoscopy. In addition, significant epithelial hyperplasia was recorded in four of 23 stent cases (17.4%), all of which involved BDS. No severe adverse events were noted.
Efficacy and tolerability of biodegradable stents for recurrent benign oesophageal strictures: The Leeds experience
N. Rabb, H. Procter, N. Burr, V. Appleby, S. Everett
25th UEG Week 2017; Oct. 28-Nov.1 – Barcelona; Spain
20 patients with 37stents were included. 30 day adverse events included 4 (11%) stent migrations and 12 (32%) with significant pain, 3 patients requiring in-patient pain control (<3 days). There were no significant bleeds or perforations.12 months following first EBS insertion 18(90%) required further endoscopic intervention due to recurrent symptoms. There was a significant reduction in median number of interventions in the 12m following EBS insertion compared to the preceding 12m (2 vs. 7 respectively, p=0.0003). Repeated EBS insertion appears a reasonable strategy for the most resistant strictures.
Single and sequential biodegradable stent placement for refractory benign esophageal strictures: a prospective follow-up study
M. M. C. Hirdes, P. D. Siersema, P. G. A. van Boeckel, F. P. Vleggaar
Endoscopy. 2012 Jul;44(7):649-54.
In total, 59 stents were placed in 28 patients. All patients had previously been treated with at least 10 dilations within 6 months; eight patients (29 %) had also been treated previously with placement of one or more SEPS or SEMS. After initial stent placement, the median dysphagia-free period was 90 days (range 14–618 days). Clinical success (absence of dysphagia ≥6 months after stent placement) was achieved in seven patients (25 %) and major complications occurred in eight patients (29 %). Three patients are currently dysphagia-free. After placement of a second biodegradable stent, the median dysphagia-free period was 55 days (range 25–700 days) and clinical success was achieved in 15% of patients. Three patients are currently dysphagia-free. After placement of a third stent, the median dysphagia-free period was 106 days (range 90–150 days), but none of the patients was clinically dysphagia-free. A possible explanation for the high complication rate may lie in the large diameter of the biodegradable stent. In seven of eight patients with major complications a 25-mm-diameter stent with 31-mm flares was placed. Conclusion: Placement of a single biodegradable stent is only temporarily effective in the vast majority of patients with RBES treated in a tertiary referral center. Sequential stenting may however be an option to avoid serial dilations.
The role of biodegradable stents in the management of benign and malignant oesophageal strictures: A cohort study
Stephen McCain, Scott McCain, Barry Quinn, Ronan Gray, Joan Morton, Paul Rice
The Surgeon (2015), http://dx.doi.org/10.1016/j.surge.2015.01.002ge.2015.01.002
17 stents were inserted to 9 patients for benign disease. Of the 9 patients who underwent a total 18 attempts at BD stenting for benign strictures, 5 were symptom free at follow-up. 4 patients with benign disease required re-intervention with a BD stent. 1 patient had 5 BD stents inserted at different time points, 1 patient had 3 BD stents inserted and 2 patients had 2 stents inserted. Median re-intervention time was 260 days (range 91-525). This study has shown BD stenting to be a very efficacious method of symptomatic relief of oesophageal stricture induced dysphagia, resulting in a significant improvement in dysphagia score post-stenting. BD stenting has an excellent safety profile, with no major complications and no stent related mortality. It would appear to offer patients with benign disease greater than 50% possibility of long-term symptom resolution. For those who require re-intervention, the duration of absence of symptoms and re-intervention time is significantly longer than would be expected with either dilatation or SEMS or SEPS.
HV Stent Plus
ELLA-HV anti-migration stent demonstrates superior performance for cancers of the gastrooesophageal junction
Mercer J, Najran P, Edwards DW, Borg P, Mullan D, Bell J, Laasch H-U
BSIR Annual meeting, Birmingham, 1st to 3rd Nov 2017
79 stent procedures were identified. There was 100% success in placing the stent across the GOJ and no reports of failure at 24 hours. Median follow up was 75 days (7-452). 4/79 (5.1%) stents migrated distally (95% CI: 1.6-12.7%), compared with 109/615 (17.7%) stent in ROST 2 registry (95% CI: 14.7-20.7%). Reduced distal migration was observed (5% significance level, p = 0.004), with a corresponding reduction in migration rate of 71.4%. The Ella HV stent confers a statistically significant reduction in distal migration compared with stent types recorded in the national registry, reducing the need for re-intervention with associated risks and cost.
A comparison of oesophageal self-expanding metal stents and their complications
C. Davidson, C. Rutherford, J. Allan, G. Simpson, J. Gray. (2016)
Royal Alexandra Hospital, GGC NHS Trust, Glasgow, UK. International Journal of Surgery. 36. S88. 10.1016/j.ijsu.2016.08.294.
A total of 98 SEMS were inserted, of which the most common were XS Ella and Niti S, with 54 and 22 inserted respectively. The remaining stent types were either not documented (19), metal (1), Ultra flex (1) or Boston Scientific Polyflex (1). Complications included blockage (total 6/98; XS Ella 2/54; Niti S 2/22) migration (total 15/98; XS Ella 0/54; Niti S 4/22) perforation (total 4/98; XS Ella 0/54: Niti S 2/22) and pain (total 7/98: XS Ella 2/54; Niti S 2/22).
Fully covered stents versus partially covered stents for palliative treatment of esophageal cancer: Is there a difference?
J.O. Alonso Lárraga, D.Y. Flores Carmona, A. Hernández Guerrero, M.E. Ramírez Solís, J.G. de la Mora Levy y J.C. Sánchez del Monte
Departamento de Endoscopia Gastrointestinal, Instituto Nacional de Cancerología, Ciudad de México, México Recibido el 30 de septiembre de 2016; aceptado el 5 de julio de 2017
A retrospective study was conducted on patients with inoperable esophageal cancer treated with self-expandable metallic stents. The 2 groups formed were: group A, which consisted of patients with a fully covered self-expandable stent (SX-ELLA®), and group B, which was made up of patients with a partially covered self-expandable stent (Ultraflex®). Group A – Technical success was achieved in 100% (n = 21) of the cases and clinical success in 90.4% (n = 19). Group B – Technical success was achieved in 100% (n = 29) of the cases and clinical success in 89.6% (n = 26). Complications were similar in both groups (33.3 vs. 51.7%) and included beside others early migration (3/21 vs. 3/26), late migration (1/21 vs. 2/26), obstruction by tumour (0/21 vs. 5/26).
Insertion of Removable Self-Expanding Metal Stents as a Treatment for Postoperative Leaks and Perforations of the Esophagus and Stomach
Inbar R, Santo E, Subch AE et al.
Israel Medical Association Journal; 2011; 13: 230–233.
Between June 2009 and February 2010 the SX-ELLA esophageal stent was inserted in five patients. The indications for stent insertion were postoperative leak in four patients and postoperative esophagopleural fistula in one patient. Three of the patients had a leak at the gastroesophageal junction following laparoscopic sleeve gastrectomy. There were no documented complaints in any of the patients regarding dysphagia or chest pain. Stents were removed electively after 6 to 7 weeks.
Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer
Pellen M. G. C., Sabri S., Razack A. et al.
Diseases of Esophagus; 2012; 25, 48–53.
Sixteen patients underwent esophageal stenting during neoadjuvant therapy. Tumors were located in the lower one third of the esophagus. Stent migration was anticipated in response to tumor regression. Patients were counseled that this was a potential outcome and slippages were readily retrieved endoscopically or at operation. Stents migrated during neoadjuvant therapy in 7/16 (43.8%) patients. Outcomes in our series suggest that SERMS could be considered a safe and feasible alternative methodof maintaining nutritional supplementation during neoadjuvant chemotherapy for stenosing esophageal cancers.
Danis Stent
Esophageal Balloon Tamponade Versus Esophageal Stent in Controlling Acute Refractory Variceal Bleeding: A Multicenter Randomized, Controlled Trial
Escorsell, Àngels, et al.
Hepatology, 2016 Jun;63(6):1957-67. doi: 10.1002/hep.28360. Epub 2016 Jan 14
28 patients were included – Esophageal Stent (n = 13), Balloon Tamponade (n = 15). Esophageal metal stents are more effective than balloon tamponade for the temporary control of massive or refractory esophageal AVB in patients with cirrhosis. The results of our multicenter randomized, controlled trial (RCT) show that the use of self-expandable esophageal stents provides a better balance of benefits and harms than balloon tamponade.
SX-Ella Stent Danis Effectively Controls Refractory Variceal Bleed in Patients with Acute-on-Chronic Liver Failure
Maiwall, R., Jamwal, K.D., Bhardwaj, A. et al.
Dig Dis Sci 63, 493–501 (2018). https://doi.org/10.1007/s10620-017-4686-8.
Acute-on-chronic liver failure patients (n = 88, mean age 47.3 ± 10.9 years) with refractory variceal bleeds received either Danis stent (Gr. A, n = 35) or continued with repeat endotherapy and vasoactive drug (Gr.B, n = 53). Control of initial bleeding was significantly more in the Danis stent group as compared to controls in both pre-match (89 vs. 37%; p < 0.001) and PRS-matched cohorts (73 vs. 32%; 0.007). Further, bleed-related death was also significantly lower in Danis groupas compared to controls in both pre-match (14 vs. 64%; p = 0.001) and PRS-matched cohorts (6 vs. 56%; p = 0.001). In a multivariate competing risk Cox model, patients who underwent DE stenting had reduced mortality in both pre-match (p = 0.04, HR 0.36, 95% CI 0.13–0.96) and PRS-matched cohorts (p < 0.001, HR 0.21, 95% CI 0.08–0.51). These findings highlight that Danis stent could be considered as the first-line management option for patients with ACLF with refractory variceal bleeds wherein TIPS is contraindicated.
A self-expanding metal stent for complicated variceal hemorrhage: experience at a single center
Wright G, Lewis H, Hogan B et al.
Gastrointest Endosc. 2010 Jan;71(1):71-8.
10 patients with variceal hemorrhage with contraindications to TIPS insertion or BT were followed. Stent insertion was successful in 9 of 10 patients. Insertion of the SX-Ella DANIS stent in patients with refractory variceal bleeding or complications of previous therapy is effective for the control of bleeding. In selected patients, SX-Ella DANIS stent insertion offers an alternative to other methods of salvage such as BT and TIPS and could be considered a substitute for BT after a prospective trial.
Treatment of Esophageal Variceal Hemorrhage with Self-Expanding Metal Stents as a Rescue Maneuver in a Swiss Multicentric Cohort
Fierz FC, Kistler W, Stenz V et al.
Case Rep Gastroenterol. 2013;7:97–105.
The use of variceal stenting in 7 patients with a total of 9 bleeding episodes in three different Swiss hospitals is described. Insertion of the stent led to immediate bleeding control in 89% (8/9) of patients. In all of these 8 cases no re-bleeding was observed subsequently while the stent remained in situ. Thanks to their safety and easy handling, SEMS are an interesting alternative to balloon tamponade as a bridging intervention to definitive therapy including the pre-hospital setting.
Self-Expanding Metal Stent (SEMS): an innovative rescue therapy for refractory acute variceal bleeding
Kinesh Changela, Mel A. Ona, Sury Anand, Sushil Duddempudi
Department of Gastroenterology, The Brooklyn Hospital Center, New York, USA. 2014.
At present, 103 cases have been described in the literature. Studies have reported 97.08% technical success rates in deployment of SEMS. Most of the stents were intact for 4–14 days with no major complications reported. Stent extraction had a success rate of 100%. Successful hemostasis was achieved in 96% of cases with only 3.12% found to have rebleeding after placement of SEMS. Stent migration, which was the most common complication, was observed in 21 % of patients.
